

Knowledge Highway
Espanol


Knowledge Highway
Espanol
What is Soil...
Soil (dirt) is a substance residing where we do not want it to reside. For example, a glass of milk versus milk on the floor. Milk in the glass is considered as being nutritional; on the floor it is considered as only soil. When it is soil, it needs to be removed (cleaned) from the surface.
What is Cleaning...
Cleaning is the process of moving soil from the "dirty" areas to where its residing is of no concern to us (down the drain). Successful cleaning is achieved when the surface is rendered free of any soil residue.
What is pH...
pH is a scale for measuring the strength of an acid or alkaline solution. For cleaning purposes, pH is used to determine the type of soil and then as a guide for selecting the correct cleaning agent.
The scale 0 to 14 indicates pH values for the intensity of acid or alkaline in a water solution.
Vegetable oil, grease, fat, petroleum lubricants, etc. will not mix with water and therefore cannot have a pH value.
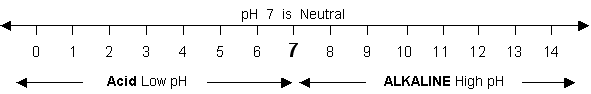
High pH soil...
High pH soil falls in the range of 7 to 14 and is called alkaline soil. The closer to 14 a solution tests from 7, the greater is its strength. A few high pH soils are: 1.) rust, 2.) lime deposit, 3.) laundry detergent film, 4.) bath/bar soap, 5.) hard water deposit. i.e. 8 is stronger than 7, etc.
Low pH soil...
Low pH soil falls in the range of 7 to 0 and is called acid soil. A common acid soil is the "dirt" we track into our buildings from outdoors. The lower a pH reading is from 7, the greater is its strength. i.e. 6 is stronger than 7, 5 is stronger than 6, and 0 is the strongest.
Determining the pH of soil...
A common method is to wet the soil with drinking water (distilled water may be closer to 7) and dip litmus paper into the solution. The paper will turn red in low pH soils and blue in high pH soils. The litmus paper will show shades of blue or red for a more accurate pH reading.
Determining the pH of a detergent...
Dip litmus paper into the concentrated detergent and make the reading. You may also wish to take the pH reading of the diluted detergent for an accurate reading of the cleaning solution.
Cleaning process overview...
Cleaning cost is 95% mechanical action (labor) and 5% chemical action. Optimum performance is required from both actions. The focus of Total Quality Housekeeping® is to get the job done using the fewest number of the best-performing chemicals consuming the least amount of labor.
How soil is held to a surface...
Soil may be held to a surface in two ways; by static electricity and/or by soil binders. An acid car battery and an alkaline flashlight battery store electricity even though they are on opposite sides of the pH scale. Since soils are either acid- or alkaline-based, they too hold electricity. The greater the soil buildup, the greater is the presence of static electricity to cause soil to cling to a surface.
Soil binders (petroleum oil and grease, animal fat or oil, and vegetable oil or shortening) act as a "glue" to hold particles of soil together and to a surface. Binders will not mix with water and will not register a pH reading; therefore, binders may be found in either high pH or low pH soils. i.e. Auto grease soil on a shop floor is acid soil; when the mechanic takes a shower the same greasy soil becomes an alkaline soil (bar soap) on the bathroom shower tiles and in the grout.
The cleaning process...
If the soil is high pH, use a low pH detergent. If the soil is low pH, use a high pH cleaner. The cleaning solution will neutralize the static electric charge in the soil to prevent the soil from reattaching to the surface being cleaned. Therefore, neutral detergents will not clean as well as detergents with sufficient pH. Neutral detergents may cause soils to become built up on baseboards, floors, grout, etc. and create visually-offensive, unsafe, and odor-emitting conditions.
Next, the detergent needs to be able to penetrate and dissolve (emulsify) the soil binder into microscopic particles. The dissolved binder particles need to hold onto the particles of soil. For example, oil in a dust mop holding onto soil particles. Hot water melts the binder and causes the soil particles to be released into the cleaning solution. The advantage of cold water cleaning is that it forces binders to hold on to soil particles while the detergent in the water encapsulates the soil until disposal. Not all detergents are formulated with the same quality of binder dissolvers.
Cold-water cleaning works best...
When hot water is used, the melted soil binder will follow the heat energy as it transfers from the hot water into the colder surface being cleaned. The melted binder will spread with the water and cover the surface being cleaned with an invisible oil-film that will attract the loose soil particles floating in the water.
The light, transparent film quickly collects new soil and becomes unsanitary. In cold water cleaning, the binder holds onto the soil and float to the top of the cold water. Gabriel formulates Fast-1-2-3 (high pH) and Walls ‘N All (low pH) detergents with the purest and highest quality ingredients to ensure squeaky-clean results without damaging the surface.
Understanding Odor Elimination, Sanitizing, and Disinfecting...
Before you can make a surface germ-free, it must be made soil-free.
Always refer to your sanitizer or disinfectant product label for usage directions and the list of organism(s) the product will control.
Click
here
to return to Art of Cleaning opening page.
Click
here
to return to Ultra Gloss "Wet Look" opening page.
Click
here
to return to Carpet Cleaning opening page.
Copyright © 2013 Gabriel First Corp. TQH and Total Quality Housekeeping are Registered Trademarks ® of TQH Systems Corp.